
In a science laboratory, the choice of the materials used can have a significant impact on analysis results. One of the materials most commonly used for laboratory instruments is borosilicate glass.
Unique Properties of Glass
Steroglass glassware is made from a low alkali borosilicate 3.3 composition. It has no magnesium, calcium or zinc, with only trace amounts of heavy metals. Borosilicate glass has a number of unique properties that make it ideal for use in a science lab. It is an inert material, which means it does not react with most of the chemicals used in labs. Furthermore, glass is transparent, allowing one to easily see what is happening inside a container, such as a beaker, flask or test tube.

Advantages of Borosilicate Glass
Chemical Resistance
Steroglass borosilicate 3.3 glassware is extremely resistant to neutral and acidic solutions, including chlorine, bromine, iodine, and other organic substances. Its greater chemical resistance is greater than that of most metals and other materials, even during prolonged reactions at temperatures above 100°C. This glass can be subjected to repeated dry and wet sterilization cycles without showing signs of surface deterioration or subsequent contamination. However, hydrofluoric acid, very hot phosphoric acid, and alkaline solutions can increase the surface attack at high concentrations and temperatures.
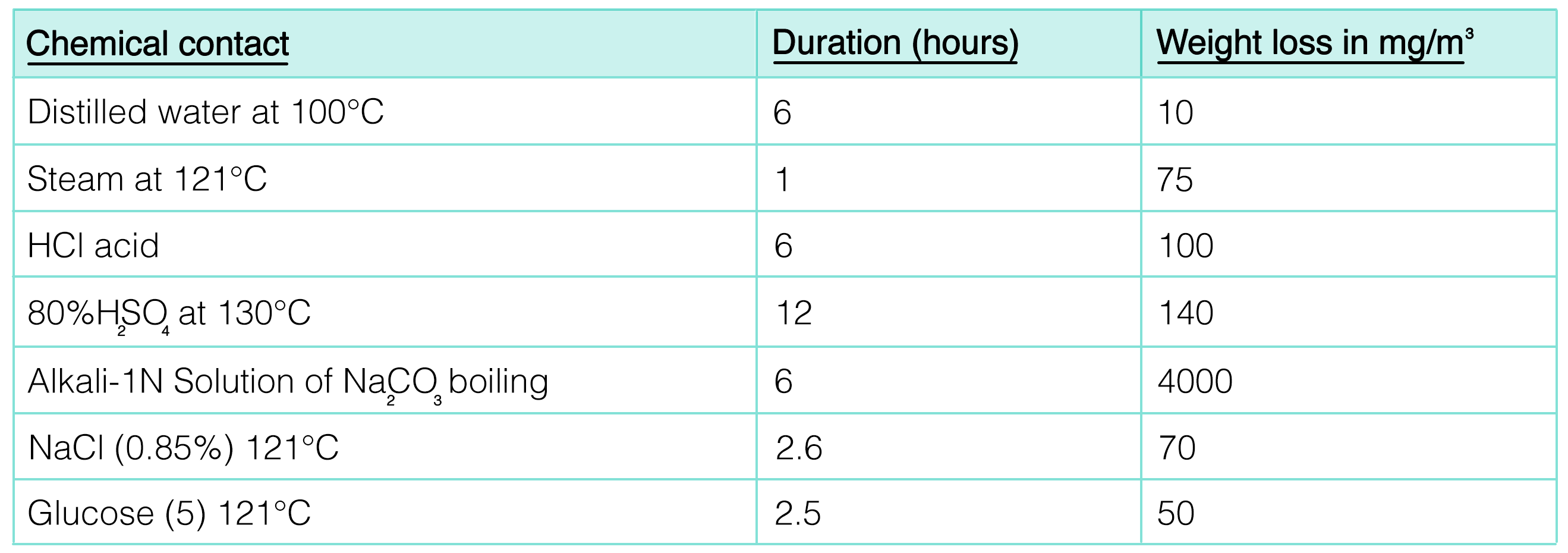
Stability and Inertness
Unlike many other materials, glass does not react with most chemicals. This makes it possible to store samples without risk of contamination or chemical alteration.
Transparency
The transparency of glass is another key advantage. Laboratory personnel can directly observe chemical or physical processes taking place inside glass containers, such as color reactions or changes in physical state. Transparency also facilitates the precise measurement of liquids.
Heat Resistance
Because Steroglass borosilicate 3.3 glass has a low coefficient of thermal expansion, the thermal stress at a given temperature gradient is low. Glass therefore has high heat resistance, allowing it to be used in a wide range of laboratory applications requiring heating or sterilization.
Linear coefficient of thermal expansion 32.5 x 10-7°C
Deformation point 515°C
Annealing point 565°C
Softening point 820°C
Specific heat 0.2
Thermal conductivity (Cal/cm3/°C/Soc) 0.0027
If heated above 500°C, glass may acquire a permanent residual stress upon cooling. Steroglass laboratory glassware is annealed under strictly controlled conditions in modern kilns, guaranteeing a very low residual stress.
Reusability and Sterilization
Glass can be cleaned and sterilized several times without losing its properties, making it a sustainable, ecological material. This characteristic is particularly useful in laboratories that must maintain sterile environments.
Easily Formed
Thanks to its low expansion and ease of forming, Steroglass 3.3 borosilicate glass can be shaped directly in the laboratory to create complex, customized applications.
In conclusion, despite the emergence of new materials, glass remains a reliable and valuable choice for analytical laboratories worldwide. It is important, however, to always follow the instructions for working safely with borosilicate 3.3 glass, including the correct procedures for heating and cooling, mixing and agitation, care with vacuum and pressure use, and adequate cleaning. Using glass correctly maximizes its lifespan and ensures a safe working environment and optimal performance.